Gene Therapy Offers Hope for Hereditary Hearing Loss: Groundbreaking Research from Seoul National University Hospital

When we think of hearing loss, we often imagine hearing aids or cochlear implant surgery.
But what if a single injection could restore hearing? ????
A research team led by Professor Sang-Yeon Lee of Seoul National University Hospital and Professor Sangsoo Bae of Seoul National University College of Medicine has actually succeeded in this dream-like research.
This achievement could be a significant first step in accelerating the era of 'gene therapy tailored to the ear'.
Hereditary Hearing Loss-Causing Mutation: Hearing Recovery Proven with 'Single Gene Editing'
- World's First Confirmation of Hearing Recovery in Humanized Mice with MPZL2 Gene Mutation
- Tissue Recovery and Safety Secured with Single Injection of Self-Developed Base Editing Gene Scissors
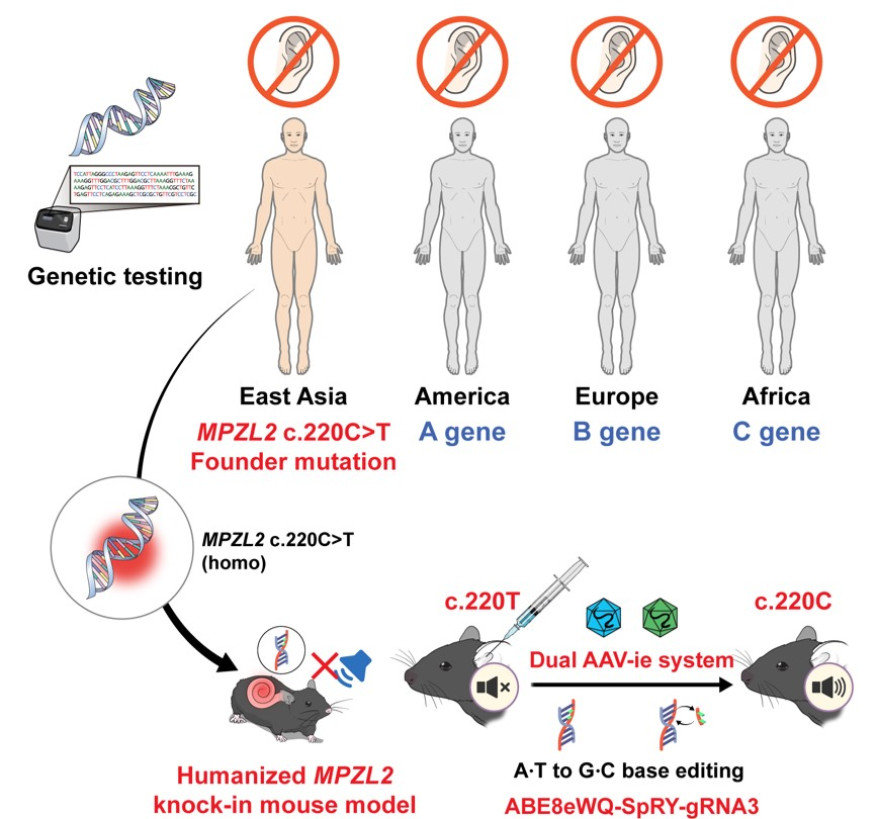
[Figure 1] Research Overview: Hearing Improvement Confirmed by Single Injection of Self-Developed Gene Scissors into Humanized Mice with MPZL2 Mutation
A Korean research team has developed a gene therapy that precisely corrects the representative mutation (c.220C>T) of the MPZL2 gene, which causes hereditary hearing loss, and has succeeded in demonstrating hearing recovery with a single injection. This study is the world's first case to demonstrate the therapeutic potential at the preclinical level by precisely applying Adenine Base Editor (ABE) to a hereditary hearing loss mutation that occurs at a high frequency in the East Asian population, representing a meaningful achievement showcasing the potential of 'one-and-done' gene therapy.
The joint research team, consisting of Professor Sang-Yeon Lee of the Department of Pediatric Otorhinolaryngology at Seoul National University Hospital and Professor Sangsoo Bae's team (So-Hyang Jung, Brain Science Cooperative Program, Han-Sol Koo, Tumor Biology Cooperative Program) of the Department of Biochemistry at Seoul National University College of Medicine, announced on the 25th that they developed a humanized mouse model with a mutation in the MPZL2 gene, which causes hereditary hearing loss, and confirmed hearing recovery and auditory cell recovery by applying self-made gene scissors.
The c.220C>T mutation in the MPZL2 gene is one of the main causes of DFNB111-type sensorineural hereditary hearing loss, occurring at a high frequency especially in the East Asian population, and is observed in about 10% of all hereditary hearing loss patients. This mutation is a nonsense mutation in which the CAG codon encoding glutamine is changed to the termination signal TAG, causing the production of MPZL2 protein to stop, leading to rapid hearing loss after adolescence and gradually progressing to profound hearing loss. However, there has been no fundamental treatment method to date.
The research team analyzed a hereditary hearing loss cohort consisting of 1,437 families from Korea and abroad, confirmed that the c.220C>T mutation is a major cause, and created a humanized mouse model that mimics this. Subsequently, they developed the latest adenine base editing gene scissors (ABE8eWQ‑SpRY) with improved editing efficiency and accuracy, and established a strategy to precisely correct the mutant base to a normal base sequence. These gene scissors work by converting adenine (A) to guanine (G) without cutting the DNA double strand, making it a next-generation gene editing technology with less cell damage and high target accuracy.
The research team loaded these gene scissors onto AAV-ie, a modified vector of adeno-associated virus (AAV), and delivered them into the ear through a single injection into the round window of the mouse cochlea. AAV-ie has high delivery efficiency to auditory cells and operates stably, and the gene scissors are guided by guide RNA (sgRNA) to accurately reach the mutation site and correct the disease-causing adenine base to normal guanine.
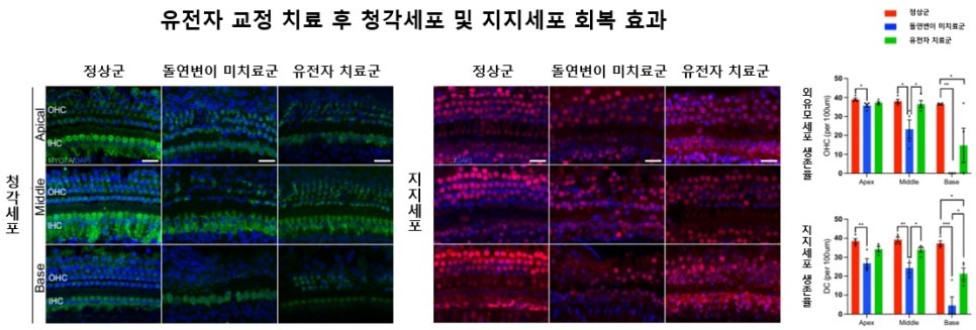
[Figure 2] Recovery Effect of Auditory Cells and Supporting Cells After Gene Editing Treatment: (Left) Significant preservation of outer hair cells (OHC) confirmed in the gene-treated group compared to the normal group (Center) Tissue structure of supporting cells (DC) also shows recovery in the treated group (Right) The survival rate of OHC and DC is significantly higher in the treated group than in the untreated group, demonstrating the recovery effect at the tissue level
The treatment effect was objectively measured through auditory brainstem response (ABR) and distortion product otoacoustic emission (DPOAE) tests. As a result, hearing improvement of 20-30dB was confirmed in all frequency bands in the treated group, and this effect lasted for more than 20 weeks. This is evaluated as an important result supporting the in vivo applicability and treatment efficiency of inner ear gene editing technology.
Tissue analysis also showed a significant increase in the survival rate of outer hair cells (OHC), which are auditory cells, and supporting cells (DC), which support them, and the tissue structure of the cochlea also recovered significantly. This suggests that gene editing contributes not only to the function of auditory cells but also to histological restoration.
To evaluate the accuracy and safety of the treatment, the research team predicted the possibility of off-target editing using the Cas-OFFinder program at the DNA level and verified that there were no off-targets through GUIDE-seq analysis. As a result of analyzing whether editing occurred in areas other than the target by sequencing the actual cochlear tissue, it was confirmed that there were no off-target effects. By performing RNA-seq analysis at the RNA level and confirming that non-target RNA editing did not occur, it was proven that the gene scissors used this time have both high target accuracy and in vivo safety.
Professor Sangsoo Bae (Department of Biochemistry, Seoul National University College of Medicine) said, "This study is meaningful in that it has practically demonstrated the possibility of precision gene therapy by accurately targeting and correcting hereditary hearing loss mutations that are frequent in the East Asian population with self-developed gene scissors."
Professor Sang-Yeon Lee (Department of Pediatric Otorhinolaryngology, Seoul National University Hospital) said, "In the past, hearing loss treatment was limited to hearing aids or cochlear implants, but in the future, we expect that a new path will open to apply personalized gene therapy to patients with hearing loss with clear genetic causes. We will continue to work towards translating this research into actual patient treatment through additional preclinical and clinical studies."
This research was supported by the Seoul National University College of Medicine Physician-Scientist Program, the Lee Kun-Hee Pediatric Cancer & Rare Disease Research Project, the National Research Foundation of Korea Excellent Young Researcher Program, and the Seoul-type Bio Project. The results of the research were published in the latest issue of 'Nature Communications (IF 15.7)', one of the top international journals.
[From left] Professor Sang-Yeon Lee of the Department of Pediatric Otorhinolaryngology at Seoul National University Hospital, Professor Sangsoo Bae, So-Hyang Jung (Brain Science Cooperative Program student), and Han-Sol Koo (Tumor Biology Cooperative Program student) of the Department of Biochemistry at Seoul National University College of Medicine
*



Source :https://blog.naver.com/chsnuh/223987558896
No comments yet.
